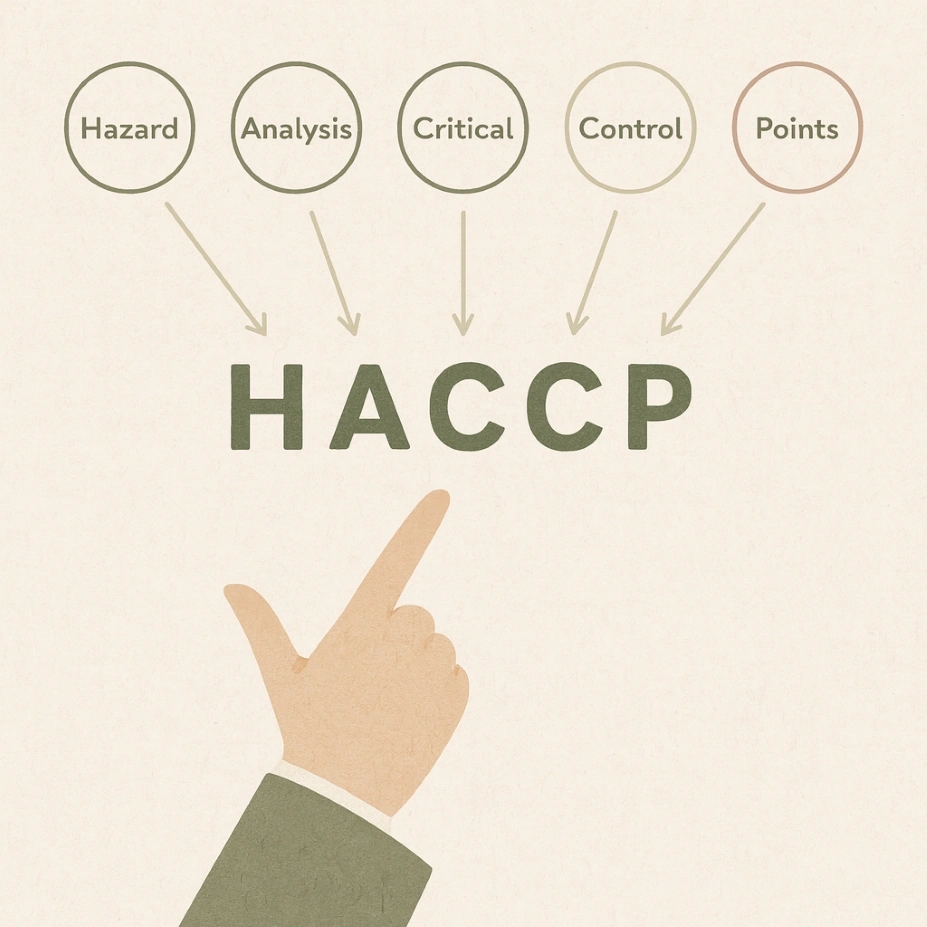
HACCP stands for Hazard Analysis and Critical Control Points — a preventive approach to food safety management that identifies, evaluates and controls potential hazards throughout the production process. Originally developed for NASA in the 1960s to ensure the safety of astronaut food, the HACCP concept has since become the foundation for modern food safety management systems worldwide. Hazard Analysis Critical Control Points is a systematic method that plays a crucial role in identifying and managing risks in food production.
Rather than reacting to problems after they occur, it focuses on designing preventive measures that protect public health and consumer trust. Food hygiene is a core component of HACCP and food safety management, ensuring that hazards are minimized and regulatory standards are met.
For manufacturers like Merywood, which produce both dietary supplements and cosmetics, a structured HACCP system helps maintain consistency and transparency in every process — from handling raw materials to packaging and distribution. By implementing a documented and verified plan, companies demonstrate commitment to high standards of food safety and regulatory compliance.
The Meaning Behind HACCP
The term HACCP stands for Hazard Analysis and Critical Control Points, and each word defines a core function of the system. “Hazard analysis” refers to the systematic identification of biological, chemical or physical food safety hazards that could compromise product safety. “Critical control points” are stages in food production where such hazards can be controlled or eliminated to an acceptable level. The goal is to ensure that risks never reach the consumer.
These food safety hazards may be occurring at any stage — during processing, storage or distribution. By analyzing every step of a process, food businesses and contract manufacturers can identify critical points where preventive controls must be applied. This is why HACCP remains the most effective method to prevent hazards before they impact quality or safety.
Why HACCP Matters for Food Businesses
For food businesses of any size — from start-ups to global manufacturers — a robust food safety management system is no longer optional. It is a regulatory requirement and a strategic asset. Implementing HACCP not only reduces food safety risks but also enhances efficiency and traceability. Food businesses are required to implement HACCP systems to comply with legal and regulatory standards. A well-designed plan builds customer confidence and aligns with international frameworks such as the Global Food Safety Initiative and ISO 22000.
Merywood applies the same methodology in the production of supplements and cosmetics, where ingredient purity and contamination control are as crucial as in the food industry. Each step of manufacturing is documented to provide clear records and auditable proof of compliance. A comprehensive HACCP program establishes critical limits at critical control points and ensures proactive control procedures are in place to meet international food safety standards. This approach not only meets regulatory expectations but also drives continuous improvement within the company.

The Seven HACCP Principles Explained
Although every HACCP plan is unique to its process, it is built on seven universal principles that define how food safety hazards are controlled. The HACCP procedure is a structured set of steps or protocols essential for effective hazard control, ensuring systematic planning and application throughout the process.
- Conduct a hazard analysis. Identify biological, chemical and physical hazards that may occur during production and handling.
- Identify critical control points (CCPs). Determine stages where control is essential to prevent or reduce risks to an acceptable level.
- Establish critical limits. For each CCP, define the maximum or minimum values (such as temperature or pH) that ensure safety.
- Set monitoring procedures. Develop methods to observe and record whether each CCP stays within critical limits.
- Establish corrective actions. Plan steps to take if monitoring shows deviation from set limits — for example, re-processing or product isolation.
- Perform verification procedures. Confirm that the HACCP system functions effectively through testing and audits.
- Maintain records and documentation. Accurate records provide documented evidence of control and compliance.
In practice, these seven principles form the core of every HACCP system — whether applied to food production, dietary supplements, or cosmetic manufacturing. They ensure that potential hazards are identified early and managed systematically, so that only safe and high-quality products reach the consumer.
How the HACCP System Works
A functional HACCP system operates as a continuous safety loop: it identifies risks, controls them, and verifies that control is effective. Each process stage is examined in detail — from receiving raw materials to packaging and distribution. The result is a clear map of food safety responsibilities across departments.
The system depends on collaboration. A multidisciplinary HACCP team is formed to determine where potential hazards may occur and which preventive measures should be in place. Their work involves developing procedures, monitoring control points, and keeping accurate records that serve as documented evidence of compliance.

KEY POINT: The effectiveness of any food safety management system depends not only on design but on routine monitoring, verification procedures, and staff awareness.
Everyone — from production operators to quality managers — is part of the HACCP process.
Developing a Written HACCP Plan
Creating a written HACCP plan is one of the most important steps for any food business. It transforms general safety principles into a customized, measurable program suited to specific products and production processes.
A written HACCP plan usually includes:
- Product description and intended use. Define how the food or supplement will be consumed or applied.
- Flow diagram of the process. Visualize every step from ingredient intake to the finished products, emphasizing that safety measures apply up to the point where finished products are ready for distribution or consumption.
- Hazard analysis. Identify potential hazards — biological, chemical, or physical — and assess their significance.
- Critical control points (CCPs). Determine where control is essential to prevent contamination.
- Critical limits and monitoring procedures. Set measurable parameters and describe how they will be checked.
- Corrective actions and verification activities. Outline what to do when a deviation occurs and how to confirm the plan’s efficiency.
- Record-keeping system. Establish how data will be stored and audited.
The HACCP team oversees developing and updating this document to ensure it remains effective as production processes evolve. When properly implemented, the plan acts as both a preventive and corrective tool — ensuring food safety hazards remain under control.
TIP: When developing an effective HACCP plan, begin by identifying steps that directly affect product integrity — such as heating, cooling, or metal detection. These are often your true critical control points.
Critical Control Points and Critical Limits in Practice
Critical control points are the stages in production where preventive actions can be applied to eliminate or reduce hazards to an acceptable level. Typical CCPs include pasteurization, filtration, pH adjustment, and the operation of a metal detector. Each CCP must have clearly defined critical limits — the maximum or minimum value that guarantees safety.
Examples of critical limits may involve temperature, pressure, time, or concentration. A critical limit is the maximum or minimum value that must be maintained at a critical control point to ensure food safety. For instance, a metal detector should reject any unit exceeding the acceptable level of contamination, while a thermal process must reach a minimum internal temperature to ensure microbiological safety.
Critical limits must be based on scientific data or regulatory requirements. They should never rely on assumptions or visual estimates alone.
Monitoring procedures ensure that CCPs stay within these defined boundaries. If a deviation occurs, corrective actions are immediately taken — adjusting process parameters, isolating affected batches, or re-testing the finished product. Each corrective action must be recorded for traceability and future verification.
Implementing HACCP Across the Food Industry
Implementing HACCP requires commitment from management and a clear understanding of the production environment. In the food industry, implementation covers facility design, staff training, hygiene protocols, and continuous verification. The goal is to integrate food safety into daily operations rather than treat it as a separate audit function.
Typical steps in implementation include:
- Training personnel on hazard awareness and proper handling.
- Validating procedures before routine use.
- Maintaining equipment sanitation to prevent contamination.
- Reviewing records and verification activities to confirm system effectiveness.
- Establishing corrective actions and communicating them across teams.
For Merywood, implementing HACCP means ensuring that every product — from functional foods to dietary supplements — meets consistent safety criteria and regulatory expectations. Preventive measures, clean production processes, and disciplined record-keeping are the pillars of reliability and customer trust.
OUR ADVICE: Begin implementation gradually, focusing on one production line or category. A well-tested pilot area allows teams to refine procedures before scaling the system to all operations.

Applying HACCP in Dietary Supplements and Sports Nutrition
HACCP principles extend beyond traditional food production. For manufacturers of dietary supplements and sports nutrition, the same systematic approach ensures purity, consistency, and consumer confidence. While supplements are not always legally classified as food, their ingredients, processes, and potential hazards are similar to those found in food businesses.
Key areas of control include:
- Raw materials. Each ingredient must be verified for origin, identity, and contamination risk. Certificates of analysis provide documented evidence of compliance.
- Production and packaging. During capsule filling or powder blending, contamination and humidity must be controlled through validated procedures.
- Storage and distribution. Environmental conditions such as temperature, humidity, and light exposure directly influence product stability.
- Handling and cleaning. Personnel hygiene and equipment sanitation prevent hazards from spreading between batches.

INSIGHT: In sports nutrition, a single contamination incident — even at a micro level — can disqualify an athlete or damage brand reputation. Preventive measures and precise records are as crucial here as in any food production line.
For Merywood, implementing HACCP in supplement manufacturing means applying the same discipline as in food safety management: controlled environments, defined critical limits, and regular verification activities. Each batch undergoes testing to ensure it meets acceptable levels for microbiological and chemical safety before packaging and release.
HACCP Principles in Cosmetic Manufacturing
Though cosmetics are not consumed as food, they still interact directly with the human body, making hygiene and contamination control equally vital. Many leading companies now adapt HACCP methodology for cosmetic manufacturing, combining it with ISO 22716 (Good Manufacturing Practice for cosmetics).
Hazard analysis in cosmetics focuses on potential contamination sources — raw materials, water quality, process equipment, and packaging. Each stage of production is reviewed to identify critical control points that may influence product safety or stability.
Main control measures include:
- Purification and monitoring of water used in formulations.
- Filtration systems preventing microbiological contamination.
- Controlled temperature and humidity in production rooms.
- Sealed packaging processes to minimize air or particle exposure.
IN PRACTICE: Merywood applies the logic of HACCP to its cosmetic division by maintaining traceable batches of raw materials and finished goods. Every step — from emulsification to filling and labeling — follows documented procedures aligned with the broader food safety management system.
This hybrid approach ensures that the same preventive culture established in supplement manufacturing supports product integrity across the company’s entire portfolio.
HACCP and the Global Food Safety Initiative
The Global Food Safety Initiative (GFSI) provides an international framework that recognizes HACCP as the foundation of every modern food safety system. GFSI-aligned schemes, such as ISO 22000, BRCGS, and FSSC 22000, all require companies to maintain a verified HACCP food safety plan as part of certification.
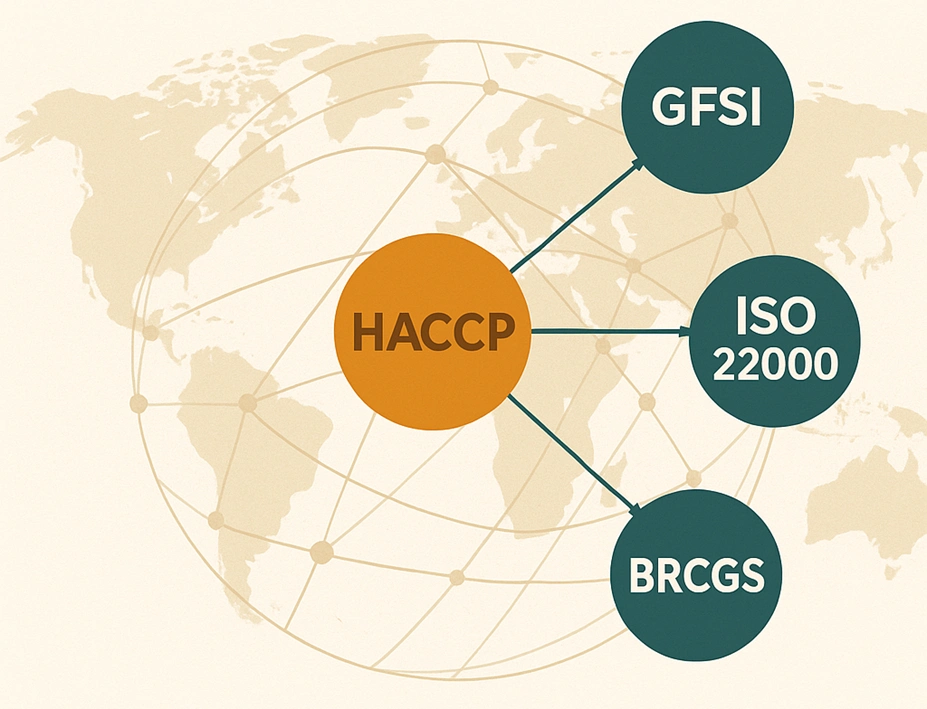
Adopting these standards allows manufacturers to:
- Harmonize compliance across markets.
- Reduce duplicate audits from different retailers.
- Strengthen brand reputation in global trade.
- Ensure consistent quality regardless of region or supplier.
NOTE: While HACCP defines what must be controlled, GFSI frameworks define how to manage and continuously improve those controls. Together they create a full food safety management system that supports regulatory compliance and consumer trust.
For Merywood, alignment with GFSI principles reinforces the company’s commitment to transparency, traceability, and sustainable manufacturing. It helps bridge communication between partners and customers who require assurance of internationally recognized safety practices.
Why HACCP Remains the Most Important Part of Food Safety Strategy
After decades of evolution, HACCP remains the most important part of any food safety program. Its strength lies in being preventive, adaptable, and evidence-based. Whether a company produces functional foods, dietary supplements, or personal care products, HACCP ensures that safety is not just a policy but a measurable process integrated into daily operations.
The system encourages continuous verification, review, and improvement. Records, audits, and performance data allow companies to identify patterns and strengthen control where it matters most. When combined with automation and digital record systems, HACCP becomes a dynamic tool for risk prediction and real-time decision-making.

For Merywood, this framework supports the company’s broader mission — delivering products that are scientifically formulated, ethically produced, and globally compliant. Each finished product represents the result of consistent monitoring, verified data, and a company-wide dedication to safety and quality.
Conclusion
Maintaining an effective HACCP system is not a one-time achievement but an ongoing process, especially for brands planning private label supplement production, where long-term consistency and audit readiness are essential. Verification procedures, internal audits, and employee training ensure that established critical limits remain valid and corrective actions remain effective. Accurate records serve as both proof of compliance and a tool for future optimization.
In today’s global market, food safety and quality assurance have become inseparable from business reputation. Companies that integrate HACCP principles into their operations — as Merywood does — not only meet regulatory requirements but also build long-term trust.

Ultimately, the goal is simple: to produce safe, reliable, and high-quality products that support public health and confidence at every stage of production.